Dr. Joachim Weber

Title: Associate Professor and Associate Chair
Education: Ph.D., Medical University of Lübeck, Germany, 1990
Postdoctoral, University of Rochester, NY, 1990-1995
Research Assistant Professor, University of Rochester, NY, 1995-2003
Research Area: Biochemistry
Office: Chemistry 232-D
Phone: 806-834-6379
Fax: 806-742-1289
Email: joachim.weber@ttuhsc.edu
Webpage: Research Group
Principal Research Interests
- ATP Synthase – The World's Smallest Rotary Motor
- Mutational Analysis of Enzymatic Function
- Biophysical Chemistry
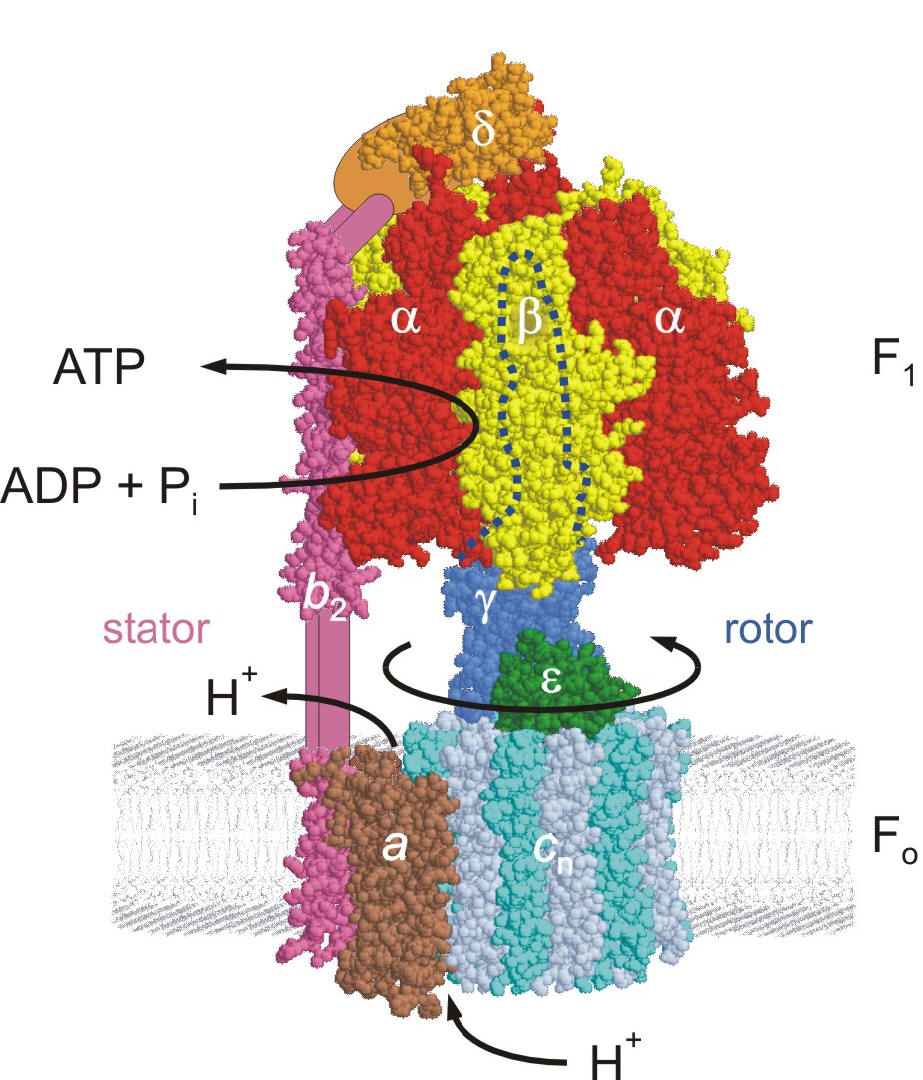
Dr. Weber's current work focuses on the question how ATP binding and hydrolysis in the catalytic site drive subunit rotation, using a variety of approaches. The applied techniques range from molecular biology (site-directed mutagenesis) to biochemistry (protein chemistry, enzyme kinetics) to biophysical chemistry (fluorescence spectroscopy) and molecular modeling. Molecular dynamics simulations and torque measurements by single-molecule analysis are performed in collaboration with other laboratories. Noteworthy recent results of this research were (a) the identification of the catalytically-active nucleotide binding site, (b) the functional analysis of a unique hydrogen-bonding network between two subunits of the enzyme, and (c) further insight into the coupling mechanism between catalysis and rotation.
Research in Dr. Weber's lab is supported by the NIH.
Representative Publications
- Kolawole, O., Millikan, C., Kumar, M., Ispas, I., and Weber, J. (2022) Microbial induced mechano-petrophysical modified properties to improve hydrocarbon recovery in carbonate reservoirs. Geomech. Energy Environ. https://doi.org/10.1016/j.gete.2022.100399.
- Kolawole, O., Millikan, C., Kumar, M., Ispas, I., Schwartz, B., Weber, J., Badurina, L., and Segvic, B. (2022) Impact of microbial-rock-CO2 interactions on containment and storage security of supercritical CO2 in carbonates. Int. J. Greenh. Gas Control 120, 103755.
- Avila-Barrientos, L.P., Cofas-Vargas, L.F., Agüero-Chapin, G., Hernández-García, E., Ruiz-Carmona, S., Valdez-Cruz, N.A., Trujillo-Roldán, M., Weber, J., Ruiz-Blanco, Y.B., Barril, X., and Garcia-Hernandez, E. (2022) Computational design of inhibitors targeting the catalytic subunit of Escherichia coli FoF1-ATP synthase. Antibiotics 11, 557.
- Mnatsakanyan, N., Park, H., Wu, J., He, X., Llaguno, M.C., Latta, M., Miranda, P., Murtishi, B., Graham, M., Weber, J., Levy, R.J., Pavlov, E.V., and Jonas, E.A. (2022) Mitochondrial ATP synthase c-subunit leak channel triggers cell death upon loss of its F1 subcomplex. Cell Death Differ. https://doi.org/10.1038/s41418-022-00972-7.
- Li, Y., Valdez, N.A., Mnatsakanyan, N., and Weber, J. (2021) The nucleotide binding affinities of two critical conformations of Escherichia coli ATP synthase. Arch. Biochem. Biophys. 707, 108899.
- Kolawole, O., Ispas, I., Kumar, M., Weber, J., and Zhao, B. (2021) Time-lapse biogeomechanical modified properties of ultra-low permeability reservoirs. Rock Mech. Rock Eng. 54, 2615 – 2641.
- Kolawole, O., Ispas, I., Kumar, M., Weber, J., Zhao, B., and Zanoni, G. (2021) How can biogeochemical alterations in shales impact caprock integrity and CO2 storage? Fuel 291, 120149.
- Swartz, D.J., Singh, A., Sok, N., Thomas, J.N., Weber, J., and Urbatsch, I.L. (2020) Replacing the eleven native tryptophans by directed evolution produces an active P-glycoprotein with site-specific, non-conservative substitutions. Sci. Rep. 10, 3224
- Mnatsakanyan, N., Llaguno, M.C., Yang, Y., Yan, Y., Weber, J., Sigworth, F.J., and Jonas, E.A. (2019) A mitochondrial megachannel resides in monomeric F1Fo ATP synthase. Nat. Commun. 10, 5823.
- Li, Y. Ma, X., and Weber, J. (2019) Interaction between γC87 and γR242 residues participates in energy coupling between catalysis and proton translocation in Escherichia coli ATP synthase. Biochim. Biophys. Acta 1860, 679 - 687.
- Mnatsakanyan, N., Li, Y., and Weber, J. (2019) Identification of two segments of the γ subunit of ATP synthase responsible for the different affinities of the catalytic nucleotide binding sites. J. Biol. Chem. 294, 1152 - 1160.
Department of Chemistry & Biochemistry
-
Address
1204 Boston Avenue, Lubbock, TX 79409-1061 -
Phone
806.742.3067
