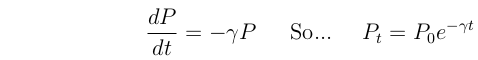
Where
 is the decay rate, t is time, P0 is the initial concentration, and e
is the decay rate, t is time, P0 is the initial concentration, and e  2.718.
2.718.
Early Earth History
Telling time
The oldest minerals so far found on earth (excluding meteorites) are around 4.2 billion years old.
The earth is expected to be older than this, though, since erosion and tectonic activity destroy rocks over time.
The oldest meteorites yield estimated ages of 4.53-4.58 billion years, with a mean of 4.54 by.
This is taken to be the age of solid material in the solar system, and thus to be the age of the earth.
Radiometric dating
Radioactive isotopes "decay" over time as particles are lost. The product is a different element.
This decay proceeds at a constant rate per unit of material.
Thus, amount of the original isotope drops
off exponentially.
For a radioactive element, P, the concentration declines as:
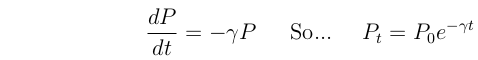
Where 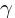 is the decay rate, t is time, P0 is the initial concentration, and e
is the decay rate, t is time, P0 is the initial concentration, and e 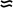 2.718.
2.718.
The plot of this function looks like this:
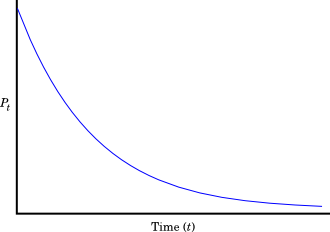
This rate often described in terms of "half
life" = time until half of the material has decayed.
Half life is related to the decay rate by:
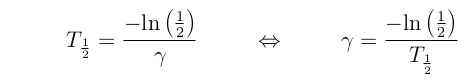
Here are some commonly used isotope pairs, and their half lives.
Example: Ar[40] is a gas which is eliminated from rock material when the rock is melted.
Thus, when we look at a solidified lava flow, any Ar[40] in the lava must have
arisen through decay of K[40].
From the amounts of Ar[40] and K[40] in the lava, and given the decay rate (calculated from physical laws and measured in the lab), we can estimate the age of the lava flow.
Checks on the accuracy of radiometric dating:
- Different isotope pairs consistently give the same ages.
- "Isochron" dating checks for contamination by looking also at another isotope of the product element (e.g. Argon in the above example).
Isochron dating
Note that there are many isotopes of each element, and only some of these are involved in radioactive decay.
For example: Strontium 87 (Sr[87]) is a decay product of Rubidium 87. However, another isotope of Strontium, Sr[86] is inert - meaning that it does not decay and nothing decays to it.
Isochron dating uses the fact that inert isotopes of an element have exactly the same chemical properties as their radioactive relatives.
Consider a parent element (P) that decays to a daughter element (D), and an inert isotope of D, called D(i). When a rock forms, the concentration of P and D may be different in different parts of the rock. However, the ratio of D/D(i) should be the same everywhere, since regions with a high affinity for D will also have a high affinity for D(i), since they have the same chemical properties.
Thus, when the rock forms, a plot of D/D(i) against P/D(i), for different samples from the rock, should look like this:
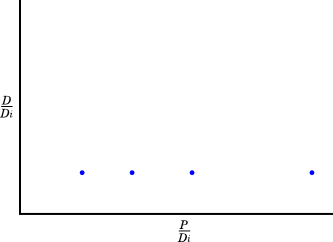
After some time has passed, each point will have moved up (because there is now more D) and to the left (because there is now less P).
The concentrations of the parent (P) and daughter (D) elements after time t are given by:
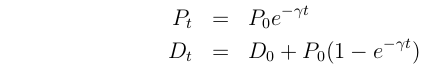
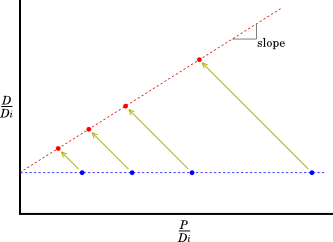
The slope of the line tells us the age of the rock.
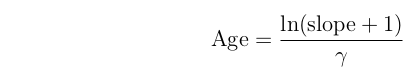
If there has been any contamination, or if some elements have leached out of the rock, or if for some reason the rate of decay was different in different samples, then the points will not lie on a line.
Isochron dating thus tells us whether or not we have good data, and then tells us the age if the data is good.
------------------------------------------------
Homework (Due Jan. 26)
Consider a parent element that decays to its daughter element with a half life of 500 million years (5*108 years).
Assume that the initial concentration of the inert isotope of the daughter element (Di) = 1, and that the initial concentration of the daughter element (D0) also = 1.
If we have four samples, in which the initial concentrations of the parent element are 1, 2, 3, and 4, respectively:
1) Draw the resulting points and the corresponding isochron line after 500 million (5*108) years have passed.
2) Draw (on the same graph) the points and corresponding isochron after 1 billion years.
------------------------------------------------
Isochron analysis of Rb-Sr concentrations in meteors looks like this
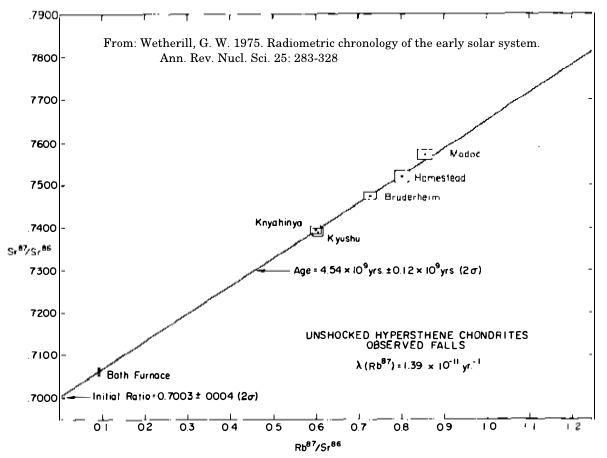
The graph above yielded the estimate of 4.54 billion years.
A related method, using isotopes of Lead with different meteorites, yields this result:

Which rocks can be dated?
Generally, only volcanic rock or isolated crystals can be dated with radiometric methods.
As a result, when we are dating biological events, we must find appropriate rocks "above" and "below" (after and before) the event and use these to get an upper and lower bound on the date for the event of interest.
For this reason, the dates that we are using in this class may change as new data is discovered from new geologic sites.
Geologic time scale - Eons
Archean
First extensive sedimentary rocks, appear around 3.5 bya.
Fossil cells appear in the 3-3.5 bya range.
Thus, life had arisen by the time of the mid Archean. Jul 8, 2021