Capturing Cancer Cells in the Act
By: Crystal Price
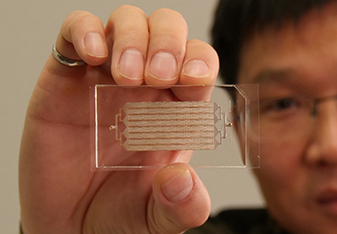
Nanoarchitecture coating incorporated into a microfluidic chip could soon be changing the way we think about cancer research and early detection. Wei Li, an assistant professor in the Texas Tech Department of Chemical Engineering, has developed a noninvasive microdevice that uses nanoarchitecture to find circulating tumor cells (CTC) within a blood sample.
CTCs are cancerous cells that escape from a primary tumor site, enter the blood stream, and adhere to a secondary site thus causing cancer to spread. Specific CTC subpopulations, rather than the whole, are responsible for cancer metastasis.
Li's goal is to isolate these subpopulations of CTCs in the blood stream in order to better understand and detect cancer progression; however, blood cells, both red and white, can also adhere to the microdevice, which can make the research more difficult. Li emphasizes that unless blood cells are dramatically decreased, it's extremely difficult to find the subgroup of tumor cells that are more aggressive.
The chip is non-invasive and requires only a simple blood draw as a sample, which is much less painful than a traditional biopsy. As the blood sample is injected into this chip, CTCs are trapped on the wall of the chip that is coated with a nanostructured biopolymer film while blood cells continue to pass through.
In theory, the chip is designed to only target the CTCs, but small quantities of blood cells still get trapped. "Blood cells still adhere because the amount is so huge, and there are electrostatic and other forces involved," Li says.
To combat this binding of blood cells, Li and his team of researchers, which includes doctors at the Henan Provincial People's Hospital in Zhengzhou, China, designed a new microchip that can be modified on the surface with a CTC-targeting antibody in the presence of anti-adhesion molecule, poly(ethylene glycol) (PEG). In the collected sample, PEG provides a dramatic decrease in the adhesion of proteins and untargeted cells thus isolating the specific CTCs.
Normally, anti-adhesion material is used underneath the antibody to capture cancer cells, though it is possible that some portion of the blood cells may also non-specifically attach to top-layer antibodies. To further enhance the purity of captured CTC samples, it is critical to change the nanoarchitecture of the films used for antibody conjugation.
Li has developed a simple solution to reduce the amount of non-specific binding of blood cells by modifying the surface of microchips with a multilayer nanofilm. The outermost layer contains both PEG for reducing blood cell adhesion and antibodies for enriching target cells.
As a result, Li dramatically reduced the non-specific binding of blood cells to approximately one out of one trillion without sacrificing the high capture efficiency. Once the blood has passed through the chip, dye is applied to see any cancer cells, or blood cells, that have bound to the film.
There are two major applications to Li's research. One is early detection of cancers present in the blood stream and the other is to identify and evaluate how and if a specific cancer treatment succeeded. These innovations could shed much needed light on how cancer spreads as well as what treatments actually eradicate cancer cells in humans.

Currently, this device has only been used with prostate and breast cancer cells because of their prevalence throughout the world, however the potential to detect other types of cancer cells is an option. "The approach is general and versatile so targeting different types of cells is a possibility, Li says. "The only thing that changes is the antibody used to attract the specific types of cancer cells. This process doesn't affect the performance of the device."
Future research based off of Li's findings could consist of molecular or genetic analysis after subpopulations of viable CTCs are released from the device. Li says that this work is important foundation work for cancer research, and, with funding by the Cancer Prevention and Research Institute of Texas (CPRIT), could be part of a larger study focusing on how to develop new ways to study CTC subpopulations using microdevices.
Li's research is featured in the November 2018 issue of Biomaterial Science and is titled "Effective Reduction of Non-specific Binding of Blood Cells in a Microfluidic Chip for Isolation of Rare Cancer Cells". Read here >>
Discoveries
-
Address
Texas Tech University, 2500 Broadway, Box 41075 Lubbock, TX 79409 -
Phone
806.742.3905 -
Email
vpr.communications@ttu.edu
