Research Areas
Adsorption models
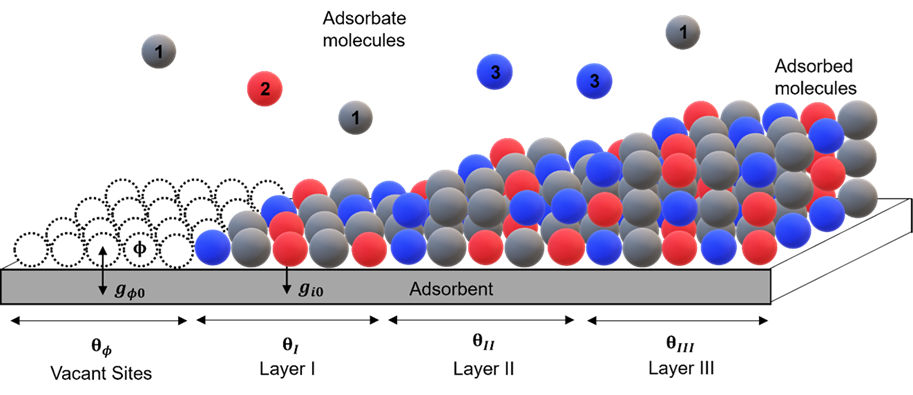
The adsorption non-random two-liquid theory, pioneered by Dr. Chau-Chyun Chen, Dr. Harnoor Kaur and his collaborators, represents a significant leap forward in modeling mixed-gas adsorption equilibria. By considering the intricate interactions between adsorbates and adsorbents, this theory offers a novel activity coefficient model that diverges from conventional approaches developed for bulk liquids. Kaur et al. accurately predict negative deviations from ideality in adsorbed phase mixtures, including complex phenomena such as azeotropic behavior observed in certain gas-adsorbent systems. With the requirement of only a single binary interaction parameter per adsorbate-adsorbate pair, this model successfully correlates a wide range of binary adsorption isotherm data. Its ability to capture the nuances of competitive adsorption makes it a powerful tool for engineers and scientists alike, facilitating precise predictions of mixed-gas adsorption equilibria.
On the other hand, the generalized Langmuir model, spearheaded by Dr. Usman Hamid and Dr. Chau-Chyun Chen, provides a comprehensive thermodynamic framework for understanding both pure component and mixed-gas adsorption equilibria. By extending the traditional Langmuir isotherm, this model accounts for competitive adsorption effects and incorporates an area-based adsorption nonrandom two-liquid activity coefficient model in its calculations. Hamid and Chen's model accurately captures dependencies on surface loading and adsorbate phase composition, offering valuable insights into the behavior of complex adsorption systems. Its validation against a variety of experimental data sets, including unary, binary, and ternary gas systems, demonstrates its efficacy in accurately representing gas adsorption equilibrium. In comparison with other models, such as the extended Langmuir isotherm and Ideal Adsorbed Solution Theory, the generalized Langmuir model consistently outperforms, further cementing its status as a vital tool in the study and prediction of adsorption phenomena.
Selected publications:
- Kaur, Harnoor, et al. "Local composition activity coefficient model for mixed-gas adsorption equilibria." Adsorption 25.5 (2019): 951-964.
- Tun, Hla, and Chau-Chyun Chen. "Isosteric heat of adsorption from thermodynamic Langmuir isotherm." Adsorption 27.6 (2021): 979-989.
- Hamid, Usman, and Chau-Chyun Chen. "Generalized Brunauer–Emmett–Teller isotherm for mixed-gas multilayer adsorption equilibria." Industrial & Engineering Chemistry Research 63.31 (2024): 13853-13863.
- Hamid, Usman, et al. "Generalization of thermodynamic Langmuir isotherm for mixed‐gas adsorption equilibria." AIChE Journal 68.6 (2022): e17663.
Eletrolyte Nonrandom Two-Liquid (eNRTL) Model
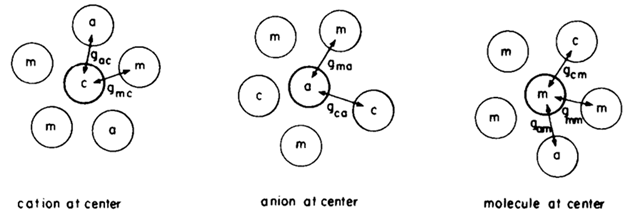
The electrolyte nonrandom two-liquid (NRTL) model, introduced by Chen et al. in 1982, is built upon several key assumptions. Firstly, it assumes that the excess Gibbs energy of a solution can be separated into two contributions: long-range electrostatic forces between ions and short-range forces between all species present. Additionally, the model assumes that the interactions between species can be described using only binary parameters, simplifying the complexity of multicomponent systems. The NRTL model provides a framework for predicting the deviation from ideality in electrolyte systems across a wide range of temperatures and concentrations. It incorporates thermodynamic principles to correlate experimental data and predict the behavior of electrolyte solutions under various conditions. This model has been particularly valuable in industrial processes where accurate predictions of solution behavior are essential for process design and optimization.
Selected publications:
- Chen, Chau‐Chyun, et al. "Local composition model for excess Gibbs energy of electrolyte systems. Part I: Single solvent, single completely dissociated electrolyte systems." AIChE Journal 28.4 (1982): 588-596
- Chen, Chau‐Chyun, and Lawrence B. Evans. "A local composition model for the excess Gibbs energy of aqueous electrolyte systems." AIChE journal 32.3 (1986): 444-454
Association Nonrandom Two-Liquid Model
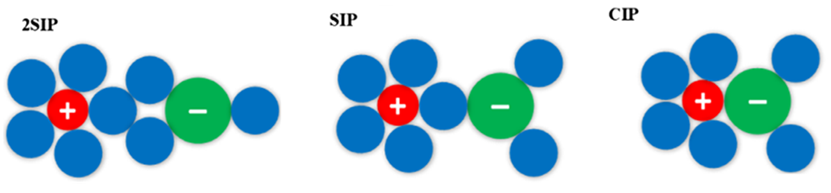
The integration of association theory into the nonrandom two-liquid model, as pioneered by Dr. Chau-Chyun Chen and Dr. Yifan Hao, represents a significant advancement in the field of thermodynamics. By refining the NRTL model to explicitly account for specific chemical associations such as hydrogen bonding, their work has greatly enhanced its predictive capability for phase equilibria in complex chemical mixtures. Moreover, their subsequent expansion of this approach to electrolyte solutions, proposed by Dr. Chau-Chyun Chen and Dr. Yu-Jeng Lin, resulting in the associaiton electrolyte NRTL (AENRTL) model, further extends its applicability to strongly associating electrolyte systems. This expansion has not only improved accuracy in predicting activity coefficients across various single-salt systems but also enabled accurate predictions for mixed-salt systems and examination of temperature dependence. With its superior accuracy over a wide concentration and temperature range, the Association NRTL and its expansion to electrolytes stand as significant contributions to the scientific community, offering a promising next-generation model for understanding and predicting the behavior of complex chemical systems.
Selected publications:
- Hao, Yifan, and Chau‐Chyun Chen. "Nonrandom two‐liquid activity coefficient model with association theory." AIChE Journal 67.1 (2021): e17061
- Lin, Yu‐Jeng, Cheng‐Ju Hsieh, and Chau‐Chyun Chen. "Association‐based activity coefficient model for electrolyte solutions." AIChE Journal 68.2 (2022): e17422
- Hsieh, Cheng-Ju, et al. "Thermodynamic modeling of aqueous lithium salt solutions with association electrolyte nonrandom two-liquid activity coefficient model." Fluid Phase Equilibria 566 (2023): 113696
Chen's Group
-
Address
1010 Boston Ave, Lubbock, TX 79409 -
Phone
806.834.3098 -
Email
chauchyun.chen@ttu.edu
