Dynamics of Small Molecule Glass Formers
Xiangfu Shi
K Z Win
Nabila Shamim
Dynamic Heat Capacity at High Pressure
We are also involved in measuring dynamic heat capacity of various polymers and liquids. We will use 3-omega technique due to Birge and Nagel (1985) which allows us to measure the relaxation spectrum of heat capacity. The approach to the glass transition has been investigated, especially at 1 atmosphere, by measuring for example dielectric susceptibility, shear compliance, and shear modulus, as a function of temperature. Recent studies of glass formers have yielded more data as a function pressure and density. The data for the heat capacity spectroscopy is relatively limited compared to other methods even at 1 atmosphere. At high pressure there is only one known dynamic heat capacity measurement by Leyser et al. in 1995. A commercial high pressure generator will be used to deliver high pressure fluid to a separate pressure vessel.

Dynamic Mechanical Properties of Imidazolium Based Ionic Liquids in the Vicinity of the Glass Transition Temperature
Recently, much interest has been focused on ambient temperature liquids that consist of cations and anions. They are widely known as room temperature molten salts or simply ionic liquids. Ionic liquids are of interest because they can be used as replacement solvents for reactions and separations since they exhibit negligible vapor pressure and they can be designed to be environmentally benign. Therefore, investigation of dynamic properties of ionic liquids is vital for design and evaluation of their application. In this study we are examining the dynamic mechanical responses of three ionic liquids of the imidazolium family using an advanced rheometric expansion system (ARES, TA Instruments) in the vicinity of the glass transition temperature. Dynamic storage modulus at several temperatures and the Time- temperature master curve near the glass temperature for the [Emim][EtSO4] material are shown in Figure 1.
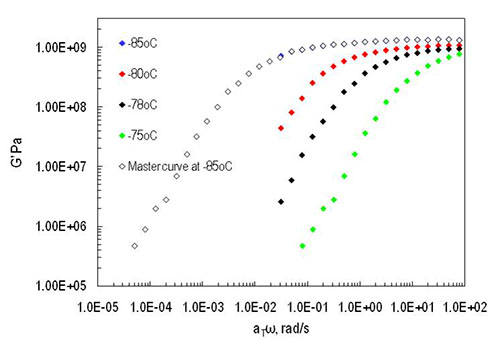
Figure: 1 Master curve for storage (G') modulus of [Emim][EtSO4] ionic liquid at reference temperature -85 °C.
Chemical Structure
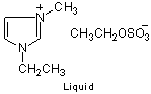
1-Ethyl-3-methylimidazolium Ethyl Sulfate [Emim][EtSO4]
Publications
Shamim, N., L. Hong, K. Hidajat and M.S. Uddin, Thermosensitive-polymer-coated Magnetic Nanoparticles: Adsorption and Desorption of Bovine Serum Albumin, Journal of Colloid and Interface Science., 304 (1), pp 1-8, 2006.
Shamim, N., L. Hong, K. Hidajat and M.S.Uddin, Thermosensitive Polymer Coated Nano-magnetic Particles for Separation of Biomolecules, Separation and Purification Technology, 53 pp 164-170, 2007.
Shamim. N, L. Hong, K. Hidajat, and M.S. Uddin, Adsorption, Desorption, and Conformational Changes of Desorbed Lysozyme from Thermosensitive Nanomagnetic Particles, Journal of Colloid and Interface Science., 320 (1), pp 15-21, 2008.
Slow Dynamics of Simple Organic Glass-forming Materials in and out of their Equilibrium
We are investigating slow dynamics of small molecule glass formers in the vicinity of their glass temperatures using mechanical spectroscopy. In the equilibrium state, dynamic and relaxational responses reveal that thermal-rheological simplicity holds b of the stretched exponential KWW function. We have examined m -toluidine, glycerol and sucrose benzoate and determined both the dynamic and viscous response over a range of temperature near to Tg.
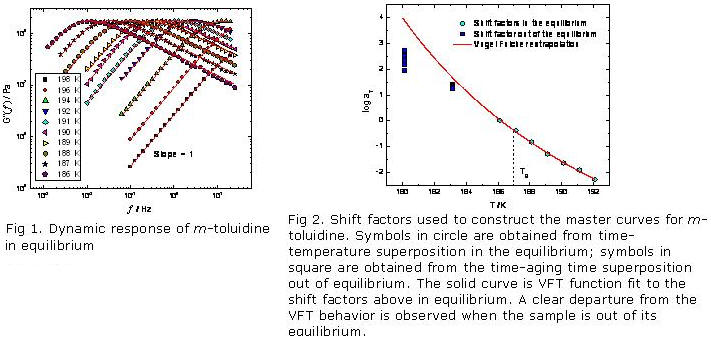
Further, we are examining the non-equilibrium state, isothermal physical aging experiments at different glassy structures. The experiment reveal that the effect of the aging process on the mechanical shear relaxation in these simple glass formers is similar to that observed in polymeric and other systems. At very long aging time, equilibrium is achieved and we observe departure from the Vogel-Fulcher-Tamman (VFT) behavior for these equilibrated samples.
Figures 1 and 2 reflect the dynamics of m-toluidine in and out of its equilibrium state.
Funding
- Petroleum Research Fund
- National Science Foundation
Polymers and Condensed Matter Physics Group
-
Address
P.O. Box 43121, Lubbock, TX 79409−3121 -
Phone
806.742.3553 -
Email
che@ttu.edu
