Research
1. Biomechanics in Cardiovascular Disease.
The goal of this work is to unravel the mechanism underlying the contribution of cellular mechanics to the development of cardiovascular disease.
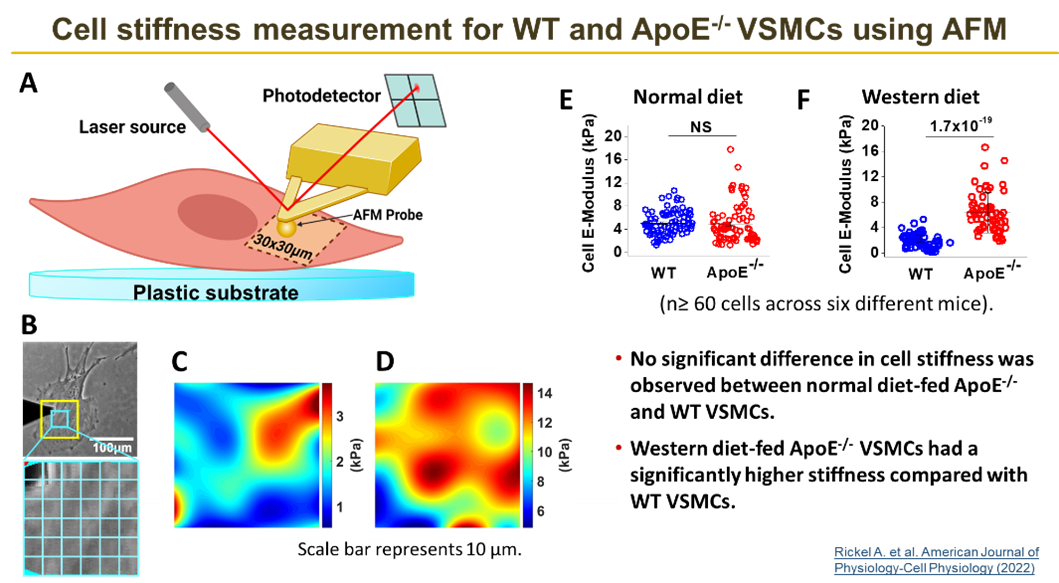
Vascular smooth muscle cells (VSMC) are the major cellular components in the blood vessel wall. Disease and aging can alter the microenvironment of VSMCs and stimulate changes in cellular mechanical functions. However, it is not fully understood how VSMC mechanics affect the progression of cardiovascular disease. Phenotypic shifting of VSMCs plays crucial roles in disease progression and is accompanied by the differential expression of cell adhesion molecules. Changes in protein expression, subsequently, regulate VSMC contractile properties and migration. It is well known that cholesterol is primarily responsible for contributing to the development of atherosclerosis by inducing endothelial dysfunction, triggering an inflammatory response in the blood vessel wall, and stimulating fatty deposition in the foam cell at the atherosclerotic plaque. However, growing evidence suggests that the role cholesterol plays in atherogenesis not only triggers inflammation, but helps to regulate cell mechanics, spreading, and migration -- critical steps in atherogenesis.
Our study focuses on how cholesterol alters VSMC functions such as biomechanical interactions of VSMCs with the extracellular matrix (ECM) at single molecular level and VSMC migratory activity within the context of 2D or 3D environment as experienced in the vascular wall. This is critical to improving our understanding of atherosclerosis and its underlying mechanisms. The long-term goal of this research is to offer new insights into the biomechanics of the vascular system and help provide valuable clues in cardiovascular disease relating to the changes in VSMC adhesion and stiffness, thereby, assisting in developing novel therapeutic strategies.
2. Biomaterials, Tissue Engineering, and Drug Delivery.
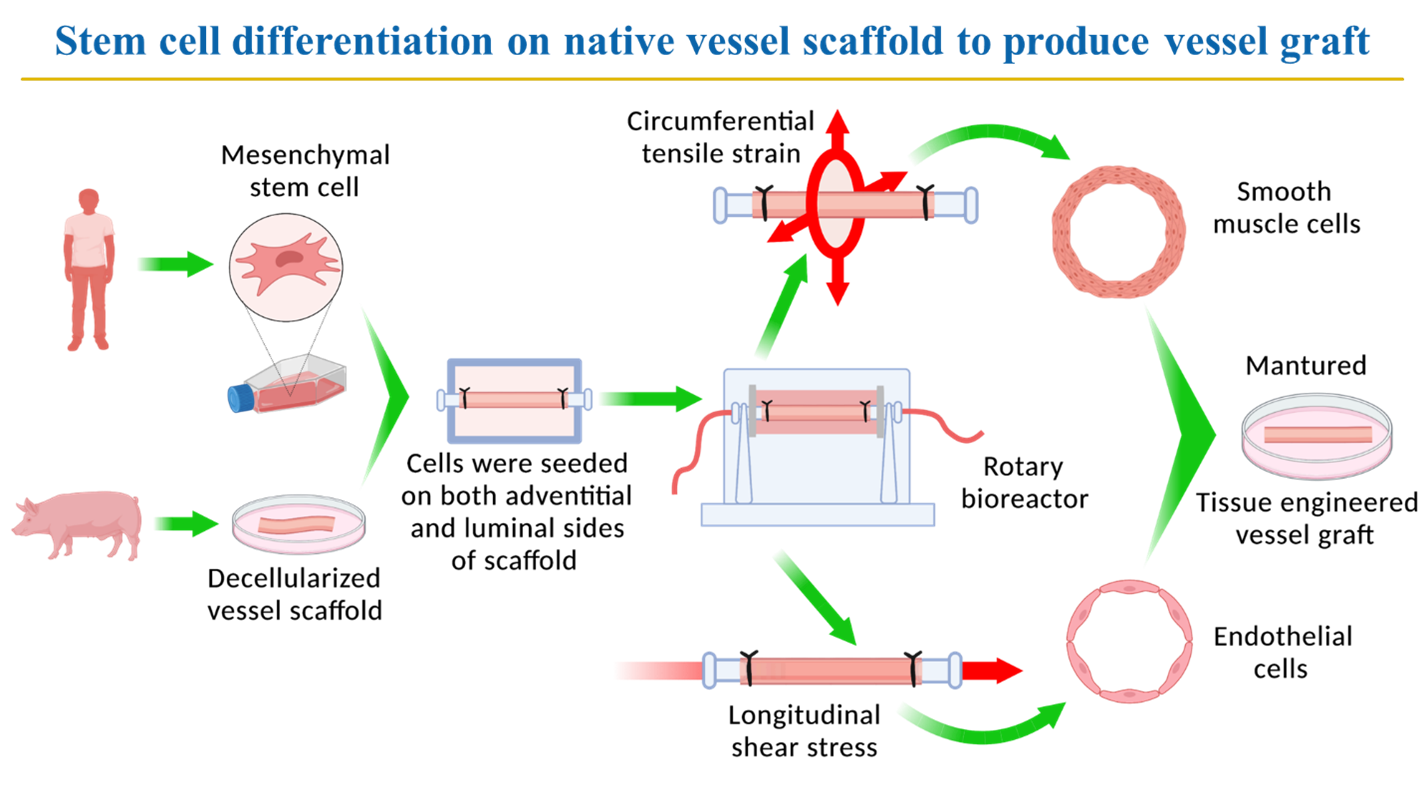
We have broad interests in biomaterials and tissue engineering, in particular, I seek to elucidate the combinatorial effects of biochemical signals and mechanical cues in stem cell derived load-bearing tissue engineering such as vascular tissue engineering and bone tissue engineering. Although significant progress has been made, creating tissue-engineered vascular grafts with replicable mechanical properties and biological functions of native blood vessels remains challenging. A scaffold with similar mechanical properties and biocompatibility to the natural blood vessel wall is ideally needed for cell attachment and proliferation to support vessel graft formation. My lab is pursuing: (1) stem cell differentiation promoted by biochemical signals and mechanical cues generated in a perfusion rotary bioreactor; and (2) biomechanical and physiological responses of vascular cells to native ECM proteins and synthetic materials for tissue engineering and drug delivery. Techniques applied in this research include atomic force microscopy (AFM), 2D and 3D cell culture system, electrospinning, fluorescent imaging systems, perfusion bioreactor, and myograph. The long-term goal is to thoroughly understand physiological responses of stem cells and vascular tissue cells to biomechanical cues and biochemical signals. This work will provide a strong theoretical foundation to develop effective therapeutic strategies in tissue engineering and drug delivery.
3. Cell Dynamics, Cell Migration, and Their Clinical Relevance.
Here, we seek to reveal the mechanism underlying cell dynamics and migration, and their relevance in cardiovascular disease and cancer metastasis. Most mammalian cells reside in and attach to ECM proteins; they constantly change their shape and mechanics through coordinated rearrangement of cellular components and position within the ECM to adapt to the variations in their environment. The long-term goal of this research is to understand the mechanism of the constant alteration in cell shape and mechanics, and their physiological function, specifically by examining the relationship between cell mechanical oscillations, cytoskeletal remodeling, and migration during the development of disease.

Hong´s Research Group
-
Address
Texas Tech University, 2500 Broadway, Lubbock, TX 79409 -
Phone
806.742.2011 -
Email
webmaster@ttu.edu
