Energetic Nanowires
M. Holtz, H. Temkin, and J. M. Berg
We are engaged in research on nanocomposite materials that exhibit energetic behavior. The nanocomposites consist of oxidizer and fuel components, which react exothermically to release a large amount of energy. Fabrication of such composites is based on a nanowire-array approach: an array of nanowires (either oxidizer or fuel) is partially embedded in a thin film (corresponding fuel or oxidizer). The wires are arranged perpendicular to the film like the bristles of a brush, allowing for maximum areal density. In our demonstration, Fe nanowires, are created in a regular array by means of electrodeposition inside nanoporous alumina membranes. This involves several processing steps, involving wet and dry etching, annealing and thin film deposition techniques to create the final product, namely Al film (fuel) attached to an array of Fe 2O 3 (oxidizer) nanowires. In the image, the diameter of a single nanowire is about 50 nm and the density of nanowires for all observed specimens is 10 E10 wires/cm^2.
We have demonstrated explosion in these nanocomposites ignited using butane flame, resistive heating element, and laser. Ignition is demonstrated by a bright flash of light. From a blackbody spectrum of the light flash, we determined the flame temperature to be ~ 4000ºC, which may be compared with theoretical value of ~3250ºC for Al-Fe2O3 reaction.
For maximum energy release, the composites must be scaled at the interdiffusion length of fuel and oxidizer, and in close physical contact. Both these conditions are fully satisfied using at the nanoscale. In our samples, the reactive part of the specimen is the portion of the nanowire embedded in the Al film. Fe oxide is effectively covered with aluminum layer of thickness 25 nm. Our sample can be considered an array of nanowires in a closely packed, monolithic arrangement. Assuming a volume reaction between Fe 2O 3 and Al we estimate that the energy released is at least 0.4J/cm 2 in our samples. This is a thousand times higher than the energy released due to a purely surface reaction (as in planar films).
The unique features of our nanocomposites are the ordered structure and tight packing, precisely controlled oxidizer-fuel sizes and positions at the nanometer scale, and intimate mechanical contact between oxidizer and fuel components. The most attractive feature of our nanocomposites lies in the energetic behavior that they exhibit at high temperature. Our nanocomposite is in effect a "nanoexplosive". Such a "nanoexplosive" can be directly integrated into novel nanodevices for applications involving triggering of explosives (as in MEMS-based fuzes), applications involving large thermal amplification and in any other application requiring light-weight, single-use energy sources.
Research Support: NSF, U.S. Army
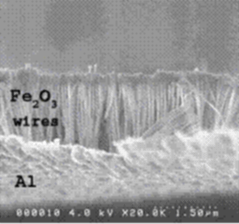
SEM image of a cross-section of Al/Fe2O3 nanocomposite.
Nano Tech Center
-
Address
Texas Tech University | Whitacre College of Engineering -
Phone
806.742.3533 -
Email
webmaster.coe@ttu.edu
