Grant Funds at Work

TTU chemist Dimitri Pappas, at right, in the lab with a chemistry graduate student.
Dimitri Pappas Is Developing Microchips That May Dramatically Cut Diagnosis Times for Sepsis & Cancer
Written by Toni Salama
In the quest to beat cancer, the urgency is to detect its presence as early as possible. Until now, that has meant scientists must know exactly which type of cancer they are looking for, then run the specific test that would identify the presence of that exact type of cancer—or eliminate it as a suspect and continue with test after test.
Chemist Dimitri Pappas compares that detection method with having to know what sort of metal the needle is made of before sifting through the haystack. Now that Pappas has received a new CPRIT High-Impact/High-Risk Research Award, the days of hunt-and-peck cancer diagnostics appear to be numbered.
Pappas, an associate professor in Texas Tech University's Department of Chemistry & Biochemistry, was awarded $200,000 over two years from CPRIT—the Cancer Prevention & Research Institute of Texas—for his grant application entitled "Microfluidic Cancer Assay for Liquid Biopsies and Early Detection." In lay terms, Pappas will be working to develop a new, quick and inexpensive method to look for the presence of cancer cells in blood and other body fluids, and it won't matter which kind of cancer he looks for.
 He describes the new method this way: "In leukemia, the presence of cancer cells in
the blood is indicative of poor outcomes. In solid tumors, cancer cells that shed
from the tumor enter the blood stream and spread to other parts of the body. The presence
of cancer cells in blood, and other bodily fluids, can be used as a diagnostic called
a 'liquid biopsy.'"
He describes the new method this way: "In leukemia, the presence of cancer cells in
the blood is indicative of poor outcomes. In solid tumors, cancer cells that shed
from the tumor enter the blood stream and spread to other parts of the body. The presence
of cancer cells in blood, and other bodily fluids, can be used as a diagnostic called
a 'liquid biopsy.'"
Specifically, the goal of Pappas' CPRIT work is to develop a new fluid microchip for early cancer detection. This chip will, he says, use an innovative antibody strategy to capture cancer cells from liquid biopsy samples, such as blood. The novelty of Pappas' work is that the antibody used to capture cells recognizes all cancer types, he says, and can be used to screen for any cancer cells that enter blood or other body fluid samples. "Unlike other methods, our microchip does not require prior knowledge of the cancer cell type," Pappas says.
That's very different from the confounding challenges of conventional detection procedures that Pappas outlines. Just look at the hurdles:
- To detect cancer cells in blood, you must first have extensive knowledge of the cancer type you are looking for.
- Next, you have to physically isolate the cancer cells from normal cells, but current methods can't always distinguish between the two.
- Then there's the messy realm of antibodies. Methods that use antibodies to capture cancer cells often miss key groups of those very cancer cells. And did we mention that there is currently no single, specific antibody that recognizes a particular cancer type?
In this project, Pappas and his research team aim to isolate leukemia, lymphoma, and tumor-based cancer cells from blood samples. And their goal is to do that with the smallest possible number of cells—the idea being that the fewer cells detected in the sample, the earlier the cancer is being caught. Along the way, they'll be determining the minimum number of cancer cells needed to detect each of these cancers in blood so that the cancer type would be revealed at the initial screen.
Pappas sees a future where doctors will use his inexpensive microchip test to cast a wide diagnostic net and, in perhaps a little as 20 minutes, learn not only if cancer is present but what type. It could be used for initial cancer screenings as well as for checking cancer patients for any residual disease. And while cancer is a serious condition for every patient who has it, Pappas believes the microchip may make a particular difference as a monitor for relapse of childhood leukemia, when treatment timing is so critical.
"This grant is really going to allow us to develop this technology at a much faster rate," Pappas says.
And this isn't the first time that Pappas has turned to microchip technology to get answers to tough diagnostic questions.
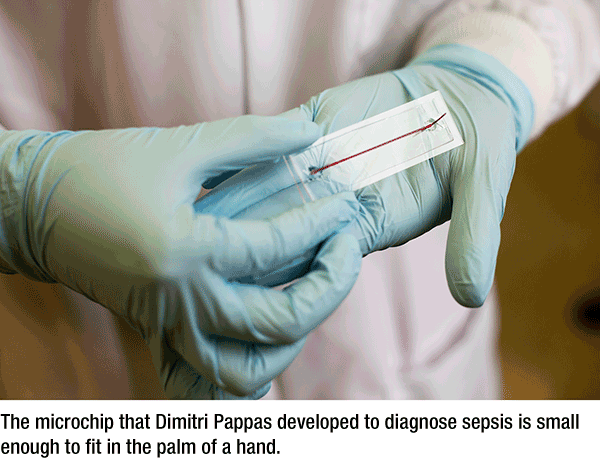
Microchip Already Works for Sepsis
Two years ago, he and graduate student Ye Zhang invented a microfluidic chip that they believed would help detect sepsis, a life-threatening blood infection. Initial tests were conducted using stem cells, with encouraging results. So their team, including Dr. John Griswold, professor and chair emeritus in the Texas Tech University Health Sciences Center Department of Surgery, conducted a clinical study using actual human blood.
And it's working. Two recently published papers from the study, one in the journal Analyst and one in Analytical Chemistry, report 98 percent accuracy in detecting sepsis with the chip. Sepsis is a potentially fatal condition in which the body's immune system goes into overdrive trying to kill a blood-borne bacterial infection.
Although sepsis is easily treatable with antibiotics, it hasn't always been easy to detect in the past. The conventional detection method—a bacterial culture to find the bacteria responsible for the infection—takes anywhere from two to 15 days. A person can die from sepsis in that time.
The chip pursues a different approach. Instead of looking for the bacteria causing sepsis, it looks for the activation of certain white blood cells, which indicate the body is trying to fight an infection. Using less than a drop of blood, the chip can detect sepsis in as little as four hours.
"Patient samples are usually where a project fails," Pappas told reporter Glenys Young for a Texas Tech Today story dated July 9. "We were able to detect sepsis and in many cases track improvement in health using patient blood. It is extremely gratifying to see the idea work so well in a clinical study."
Pappas expects to spend the next 12-18 months analyzing all the data from the clinical study, which was funded by a grant from The CH Foundation. "Ultimately, this type of work—for it to be successful—has to be commercialized," he said. "It has to be out there in the hands of physicians. We want it to be adopted outside the walls of this building and outside the city of Lubbock."
Glenys Young contributed to this story.
College of Arts & Sciences
-
Address
Texas Tech University, Box 41034, Lubbock, TX 79409-1034 -
Phone
806.742.3831 -
Email
arts-and-sciences@ttu.edu
