Leading the Way
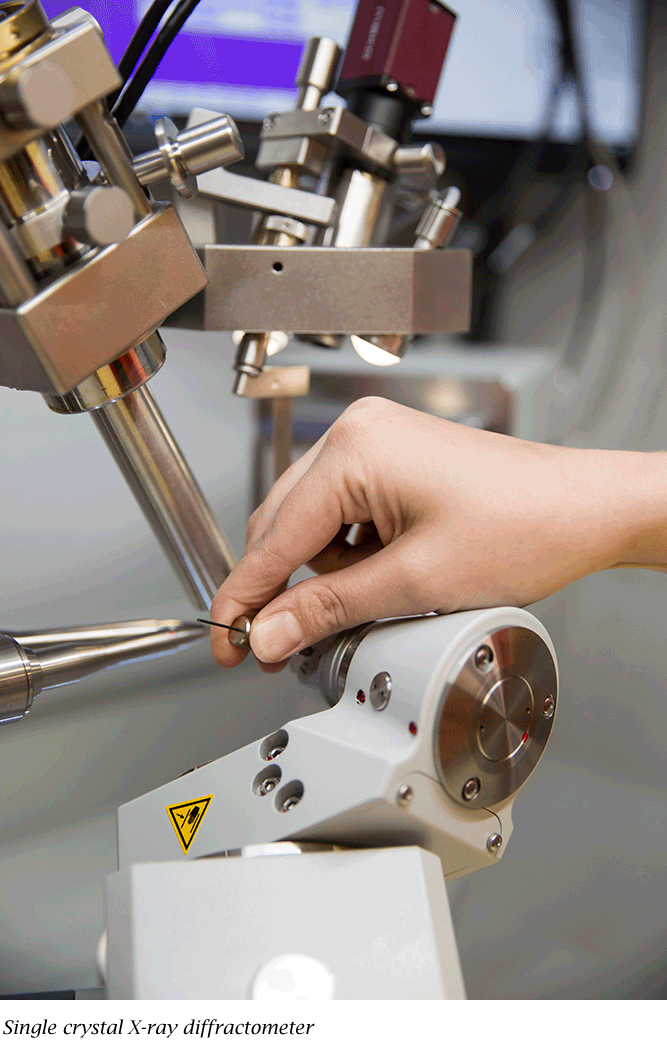
Texas Tech University chemist Kristin Hutchins at the single crystal X-ray diffractometer she uses in her research.
Chemist Kristin Hutchins Targets Ways to Remove Contaminants From Water
4.5.2019 by Glenys Young
The wonders of modern medicine are many. It has given millions of people the ability to liver longer, healthier lives just by taking a few little pills each day.
But of those few little pills each day, a trace amount leaves the body with each trip to the bathroom, not to mention the leftover medications that get flushed down the toilet or washed down the sink drain. All those trace amounts from each person, each day, can add up to a lot over time.
This wouldn't be a problem if water treatment plants were able to effectively remove all those medications before putting the water back into nature, where fish swim in it, plants use it to grow, animals drink it and people consume both the water itself and many of the plants and animals that used it before. If treatment plants could just get all that medication out, there would be no need to worry about the effects it may be having on the entire ecosystem.
Of course, that's not the case—yet. The most widely used filtration methods can only remove some medications, and some only partially.
But a Texas Tech University researcher is working to change that.
Finding a Solution
Kristin Hutchins, an assistant professor in the Department of Chemistry & Biochemistry, is leading a group of graduate students and undergraduate researchers who hope to really clean the water.
"In the literature, researchers are starting to talk about organic micro-pollutants," Hutchins said. "They're not there in high quantity, but thinking ahead, we do need to start concerning ourselves with this, and looking for a way to remove them, because we currently don't have that technology."
Hutchins and her team are working to design materials that can remove various contaminants from water, including heavy metals and pharmaceutical compounds. One research project was recently published in a virtual special issue of Crystal Growth & Design, focusing on emerging investigators.
 "We looked at one drug, specifically bezafibrate," she said. "People commonly take
it for high cholesterol. It's been found in wastewater in low concentrations, but
still there, so it's one of our targets.
"We looked at one drug, specifically bezafibrate," she said. "People commonly take
it for high cholesterol. It's been found in wastewater in low concentrations, but
still there, so it's one of our targets.
"One of our strategies is to look at the structure of this drug molecule and think about how we can make a material to capture it. And then we design that material."
Hutchins first tried bonding bezafibrate with different molecules to see which molecule would bond strongest, effectively capturing the drug. One molecule, 4-dimethylaminopyridine (DMAP), bonded with the bezafibrate more than 18 times better than other molecules she tried.
Focusing her attention to just that molecule, she grew crystals from a DMAP-bezafibrate mixture. Then, using a special piece of equipment—a single crystal X-ray diffractometer—she could visualize the results and examine how well the DMAP bonded with the bezafibrate.
"It's my favorite technique, because it generates a visual representation," she said. "You can really see it.
"We also did experiments in a liquid phase, because, ultimately, we want to capture the drug in water. We wanted to see if we see similar behavior in the solid and in the liquid phases, and we did. That one molecule just worked so much better than any of the rest."
Moving Ahead
Now, DMAP is the key ingredient to Hutchins' next step: creating a material to actually capture bezafibrate from water.
"Ultimately, it'll be a polymer, but you can think about it like a filter," Hutchins said. "You would take water, run it through and the molecule would just be at the surface to capture the drug, and you'll have clean water on the other side."
Her group is working to create and test various polymers before adding in the DMAP.
"There are a lot of things you can make the material out of, and every little change you make can change how well it works," Hutchins explained. "We're starting with a really simple kind of base for the polymer that's really inexpensive and gives it good materials properties. Then we put in a certain percentage of the molecule that's going to capture the drug. We don't think you need the whole material made of DMAP; it would be more expensive to do that, too.
"It's going to be a case of finding that right balance, making sure everything forms this nice, uniform material in which everything is evenly distributed. Our preliminary tests are looking good, but from where we are now to having something scalable, we're probably talking about years, really. You can get things to work on a lab scale, but then scaling up is always a different situation."
The scale on which she decides to focus also will determine what her filter ultimately looks like.
"It's cool to think about it like a one-on-one, tap filter addition," she said, "but it could go as big as a wastewater treatment plant-type scale."
Of course, that is likely more than a decade away. And a lot, Hutchins said, depends on how the levels of micro-pollutants in the environment change in that time.
"Going forward, people will characterize more and more of these micro-pollutants and try to see, at different stages, are they high enough now that we need to really worry about it?" she said. "For example, there have been some recent studies examining the levels of micro-pollutants in fish. This is where we may see effects first, and that could be an indicator for when action needs to be considered.
"We're probably in the parts per billion or parts per trillion levels, so we are nowhere near drinking medicines at therapeutic concentrations. But the safe levels for every contaminant is different, and we are developing and using more medications than we ever used to."
Perfect Fit
Hutchins may be uniquely qualified to conduct this research because it brings together two very different areas of chemistry from her past. After working with crystals in graduate school and polymers as a postdoctoral researcher, she knew she wanted to combine the two in some meaningful way – and now she has.
"The strength of crystals is that you can really visualize these interactions. You can see a picture," she said. "With polymers, they're robust materials that have these nice properties. So, it's a combination of, can I take the strengths of both and use the small molecules to design it and get the material that I want at the end?"
That said, working with water contaminants was never her plan.
"Environmental problems are really new for me," she laughed. "With my background, I just said, 'OK, I know how to design crystals and materials, but what can I apply this to?' I mean, we can make things just to make them, but let's try to target something. That was an area I had read about, and it was kind of sticking out in my mind.
"It's definitely a new area for me, but I think it's something that will need attention long term, and I think it's something this type of chemistry can address."
Looking to the Future
As work progresses on the DMAP-based polymer, Hutchins already is starting on her next project to remove contaminants from water—this time in the drilling industry.

"With that project, we're targeting ions that are problematic in fracking water, which is sort of a uniquely Texas problem," she said. "You get these ions that make the water unusable, so we're looking at a way to separate them out so that the water is usable after fracking."
Joining Hutchins on this are Michael Findlater, an associate professor of chemistry who specializes in organic chemistry, and Weile Yan, an associate professor in the Department of Civil, Environmental & Construction Engineering in the Edward E. Whitacre Jr. College of Engineering who specializes in the environmental applications of nanomaterials as well as groundwater and hazardous waste remediation.
"Michael and I design and synthesize the molecules and do some small-scale testing," Hutchins said, "and Weile does membrane electrodialysis. Basically, we put our polymer on the surface of a membrane, then Weile can run water spiked with a choice contaminant through it to monitor how well it's removed."
Yan says it's an important project for several reasons.
"In Texas, the fracturing industry is concentrated in areas where the fresh water supply is scarce," she said. "Our proposed method seeks to enable the use of low-quality water sources, such as brackish groundwater, for fracturing, so that competition for limited potable water supply by the energy industry can be reduced.
"On a more fundamental level, the ability to separate ions selectively—moving certain ions of interest as opposed to moving all dissolved salts in water—allows more flexible water treatment options for different applications. There are many examples in addition to fracturing water treatment, such as the removal of contaminants from bulk waste stream and doing so in a more energy-efficient manner."
Findlater said incorporating Hutchins' expertise in the synthesis of larger molecules with Yan's work and his own synthetic chemical knowledge will be invaluable in pushing the boundaries of what can be achieved with this approach.
"Incorporation of our design principles into macromolecular species will provide a more robust technology platform that we plan, ultimately, to commercialize," he said. "This kind of highly interdisciplinary science, targeting problems of national significance, has the potential to lead to truly transformative breakthroughs. We are lucky to have experts like Drs. Yan and Hutchins here on our campus to make this possible."
College of Arts & Sciences
-
Address
Texas Tech University, Box 41034, Lubbock, TX 79409-1034 -
Phone
806.742.3831 -
Email
arts-and-sciences@ttu.edu

