Dr. Richard A. Bartsch

Title: Paul Whitfield Horn Professor, Emeritus
Education: Ph.D., Brown University, 1967
Research Area: Organic, Analytical, & Polymer Chemistry
Email: dickbartsch07@gmail.com
Principal Research Interests
- Design and Synthesis of Novel Organic Molecules for Ionic and Molecular Recognition
- Separation Science
- Metal Ion Complexation and Separation by Multidentate Ligands
- Preparation and Applications of Ionic Liquids
Currently attention is focused upon the use of calix[4]arene as a scaffold for attachment of pendant acidic groups through the phenolic oxygens. For calix[4]arene, the limiting conformations of cone, partial cone, 1,3-alternate, and 1,2-alternate are shown below as structures (1-4), respectively.
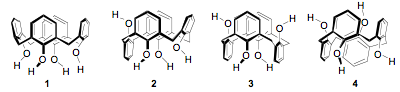
With appropriate substitutions on the four phenolic oxygens, the conformation can be mobile or locked. For example, ligand (5) is conformationally mobile and ligand (6) in which the two methyl groups in (5) have been replaced in the butyl groups is locked in the cone conformation. Ligand (7) is conformationally locked in the 1,3-alternate conformation with the two acidic groups on the opposite side of calix[4]arene framework.
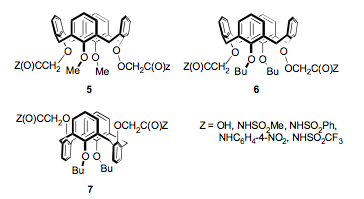
Evaluation of these ligands by solvent extraction of metal ions from aqueous solutions into chloroform reveals that the both the extraction efficiency and selectivity are influenced by the conformations of similarly substituted ligands.
Connection of two phenolic oxygens of the calix[4]arene scaffold with a polyether chain gives calixarene-crown ether ligands, also called calixcrowns. Attachment of two distal oxygens results in a calix[4]arene-1,3-crown ether ligand (8); whereas linking two proximal oxygens provides a calix[4]arene-1,2-crown ether analogue (9). (Both structures are shown with the calix[4]arene unit in the cone conformation.)
![structures are shown with the calix[4]arene unit in the cone conformation](/chemistry/Faculty/bartsch/Picture_4.png)
Attachment of two ionizable groups to the phenolic oxygens gives di-ionizable calix[4]arene-crown ethers in which the spatial relationship of the acidic side arms to the crown ether cavity is controlled by the conformation of the calix[4]arene unit. The influence of such systematic structural variations upon the metal ion complexing ability of the ligands is being probed by solvent extraction.
In a collaborative research effort with Professor Edward Quitevis of this department, fundamental features of ionic liquids are being examined. Ionic liquids are organic salts that are liquids at room temperature. These novel media are highly polar with unique solvating abilities that can be 'tuned' by structural variations within the cationic and/or anionic components. Ionic liquids such as those shown below are prepared by a Bartsch coworker for study by the Quitevis Research Group.
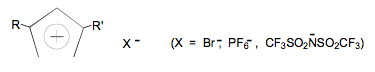
Representative Publications
- "Sorption of Heavy Metal Ions by Silica Gel-Immobilized, Proton-Ionizable Calix[4]arenes.", J. Wang; D. Zhang; T. R. Lawson and R. A. Bartsch, Talanta 2009, 78, 477-483.
- "Effect of Cation Symmetry and Alkyl Chain Length on the Structure and Intermolecular Dynamics of 1,3-Dialkylimidazolium Bis(trifluoromethanesulfonyl-amide Ionic Liquids.", D. Xiao; L. B. Hines, Jr.; S. Li; R. A. Bartsch; E. L. Quitevis; O. Russina and A. Triolo, J. Phys. Chem. B 2009, 113, 6426-6433.
- "Metal Ion Complexation in Acetonitrile by Di-ionized Calix[4]arenes Bearing Two Dansyl Fluorophores.", Ű. Ocak, M. Ocak; K. Surowiec; R. A. Bartsch; M. G. Gorbunova; C. Tu and M. Surowiec, J. Incl. Phenom. Macrocycl. Chemistry 2009, 63, 131-139.
- "Di-ionizable p-tert-Butylcalix[4]arene-1,2-monothiacrown-3 Ligands in the Cone Conformation: Synthesis and Metal Ion Extraction.", D. Zhang; J. D. Crawford and R. A. Bartsch, Tetrahedron 2008, 64, 9843-9849.
- "Di-ionizable Calix[4]arene-1,2-crown-5 and -crown-6 Ethers in Cone Conformations: Synthesis and Divalent Metal Ion Extraction.", C. Tu; K. Surowiec; J. Gega; D. W. Purkiss and R. A. Bartsch, Tetrahedron 2008, 64, 1187-1196.
- "Di-ionizable p-tert-Butylcalix[4]arene-1,2-crown-3 Ligands in Cone and 1,2-Alternate Conformations: Synthesis and Metal Ion Extraction.", D. Zhang; X. Cao; D. W. Purkiss and R. A. Bartsch, Org. Biomol. Chem. 2007, 5, 1251-1259.
- "Effect of para-Substituents on Alkaline Earth Metal Ion Extraction by Proton Di-ionizable Calix[4]arene-crown-6 Ligands in Cone, Partial-cone and 1,3-Alternate Conformations." H. Zhou; D. Liu; J. Gega; K. Surowiec; D. W. Purkiss and R. A. Bartsch, Organic & Biomolecular Chemistry 2007, 5, 324-332.
- "Efficient Divalent Metal Cation Extractions with Di-ionizable Calix[4]arene-1,2-crown-4 Compounds." C. Tu; K. Surowiec and R. A. Bartsch, Tetrahedron 2007,63, 4184-4189.
- "Synthesis and Lead(II) Sorption of Silica Gel-Immobilized, Di-ionizable Calix[4]arenes." D. Zhang; J. Wang; T. R. Lawson and R. A. Bartsch, Tetrahedron 2007, 63, 5076-5082.
- "Novel Calix[4]arene-thiacrown Ether for Selective and Efficient Extraction of Ba(II), Pb(II) or Hg(II)." C. Tu; K. Surowiec and R. A. Bartsch, Journal of Inclusion Phenomena and Macrocyclic Chemistry 2007, 58, 361-366.
Department of Chemistry & Biochemistry
-
Address
1204 Boston Avenue, Lubbock, TX 79409-1061 -
Phone
806.742.3067
