Dr. Michael F. Mayer

Title: Associate Professor
Education: Ph.D., University of Wisconsin-Milwaukee, 2000
Postdoctoral Study, University of Illinois at Urbana-Champaign, 2001-2004
Research Area: Organic Chemistry
Office: Chemistry 223-C
Phone: 806-834-3689
Email: mf.mayer@ttu.edu
Principal Research Interests
- Synthetic Methodology
- Self-Assembly
- Macromolecular/Nanoscale Chemistry
- Catalysis
- Supramolecular Chemistry
- Polymers
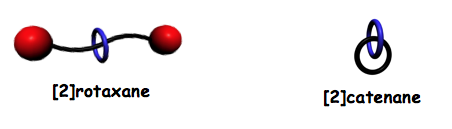
Compounds that consist of many persistently entangled individual structures are also of interest since the properties of these assemblies can be tuned through variation of the proportion of the entangled component species. Examples of persistently entangled polymeric assemblies include polyrotaxanes, polycatenanes and daisy-chain polymers.
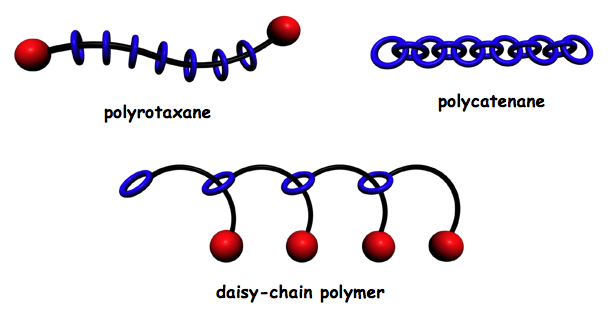
Recently, the Mayer group developed a new approach to a stable entangled assembly that is composed of a molecular chain that was threaded through numerous molecular rings by a process known as an entropy-driven ring-opening olefin metathesis polymerization (ED-ROMP).
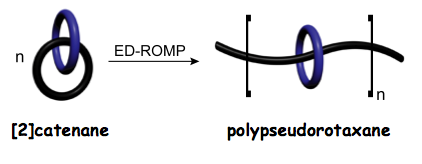
The Mayer group also recently designed and prepared a shape-dynamic molecular structure, or more specifically, a structure that was capable of sampling both self-entangled and disentangled states. The structure was designed such that it was biased to favor a disentangled state, which was approximately 50% longer than the self-entangled state. The compound was further designed such that it featured recognition sites for certain ions; when these ions were introduced, they bound the recognition sites along the ring and chain, thus tightly pulling the sites together and retaining the structure in an exclusively self-entangled state. Removal of the ions, which essentially served as linchpins, allowed the structure to re-gain its extended state and complete a chemically-induced actuation cycle, thus providing proof-of-principle of a novel nanoscale mechanical switch.
Representative Publications
- "Characterization and Photocatalytic Behavior of 2,9-Di(aryl)-1,10-phenanthroline Copper(I) Complexes," M. Mustafa Cetin, Roman T. Hodson, C. Robin Hart, David B. Cordes, Michael Findlater, Dominick J. Casadonte, Jr., Anthony F. Cozzolino and Michael F. Mayer, Dalton Trans. 2017, 46, 6553-6569. DOI: 10.1039/c7dt00400a
- "Synthesis of Metalated Pseudorotaxane Polymers with Full Control over the Average Linear Density of Threaded Macrocycles," Songsu Kang, M. Mustafa Cetin, Ruiyang Jiang, Eric S. Clevenger and Michael F. Mayer, J. Am. Chem. Soc. 2014, 136, 12588-12591. DOI: 10.1021/ja507167k
- "Actuator Prototype: Capture and Release of a Self-Entangled [1]Rotaxane," Zheng Xue and Michael F. Mayer, J. Am. Chem. Soc. 2010, 132, 3274-3276. DOI:10.1021/ja9077655
- "Asymmetric Aziridination of N-tert-butanesulfinyl Imines with Phenyldiazomethane via Sulfur Ylides," Zheng Xue, Veronica M. Dee, Louisa J. Hope-Weeks, Bruce R. Whittlesey and Michael F. Mayer, Arkivoc 2010, 7, 65-80.
- "Entropy-Driven Ring-Opening Olefin Metathesis Polymerizations of Macrocycles," Zheng Xue and Michael F. Mayer, Soft Matter 2009, 5, 4600-4611. DOI: 10.1039/b913696g
- "Tetrahydroquinoline Syntheses Induced with Catalytic Amounts of Viologen Additives," Zheng Xue, Anindya Samanta, Bruce R. Whittlesey and Michael F. Mayer, Tetrahedron Lett. 2009, 50, 6064-6067. DOI:10.1016/j.tetlet.2009.08.058
- "Polypseudorotaxanes via Ring-Opening Metathesis Polymerizations of [2]Catenanes," Songsu Kang, Brandon M. Berkshire, Zheng Xue, Manav Gupta, Christianah Layode, Preston A. May and Michael F. Mayer, J. Am. Chem. Soc. 2008, 130, 15246-15247. DOI:10.1021/ja806122r
- "Aziridine Synthesis in the Presence of Catalytic Amounts of Pyridiniums or Viologens," Zheng Xue, Arindam Mazumdar, Louisa J. Hope-Weeks and Michael F. Mayer, Tetrahedron Lett. 2008, 49, 4601-4603. DOI:10.1016/j.tetlet.2008.05.085
- "A Double Ring-Closing Olefin Metathesis Approach to [3]Catenanes," Manav Gupta, Songsu Kang and Michael F. Mayer, Tetrahedron Lett. 2008, 49, 2946-2950. DOI:10.1016/j.tetlet.2008.03.012
- "A Catalytic Synthesis of Aziridines without the Usual Byproducts," Arindam Mazumdar, Zheng Xue and Michael F. Mayer, Synlett 2007, 2025-2028. DOI:10.1016/j.tetlet.2008.03.012
Department of Chemistry & Biochemistry
-
Address
1204 Boston Avenue, Lubbock, TX 79409-1061 -
Phone
806.742.3067
