Dr. Bruce R. Whittlesey

Title: Associate Professor Emeritus
Education: Ph.D., University of Texas at Austin, 1985
Postdoctoral Study, University of Illinois, 1985-87
Research Area: Inorganic and Materials Chemistry
Phone: 806-834-3048
Email: bruce.whittlesey@ttu.edu
Principal Research Interests
- Organometallic Chemistry
- Transition Metal / Group 13 Complexes
- Electronic Materials
The second area of study involves the preparation and reactivity of mixed-metal complexes in which transition metals are attached to Group 13 elements (B, Al, Ga, In, Tl). These compounds are then examined to better understand the bonding between the transition metal and the Group 13 element as well as to explore the possibility of their development as catalysts for organic synthesis and as precursors for the preparation of new inorganic materials. A number of important industrial processes utilize transition metal compounds and Lewis acids as cocatalysts (e.g. the Dupont process for preparing adiponitrile), and it has been shown that coordination of a Lewis acid to a reactant molecule can greatly enhance its ability to insert into transition metal-carbon bonds. One of the major goals of this research is to prepare catalysts in which the Lewis acid site, in the form of a Group 13 element, is attached directly to the transition metal rather than existing as a separate species in solution.
Representative Publications
- "Terminal Coordination of a Gallium(I) Species to a Transition Metal. Syntheses and Crystal Structures of Ru{GaCl(THF)2}{GaCl2(THF)}2(CO)3.0.5 THF and Ru2 {GaCl2(THF)}2(CO)8." Harakas, G.N.; Whittlesey, B.R., Inorg. Chem.1997, 36, 2704-2707.
- "Xenophilic Metal Clusters: Preparation and Crystal Structure of {m-Mn(THF)2}2Fe2(CO)8 (THF = tetra-hydrofuran)." Harakas, G.N.; Whittlesey, B.R. J. Am. Chem. Soc.1996, 118, 4210-4211.
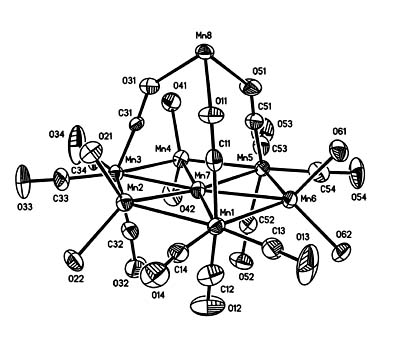
- "An Unusual Transition Metal Cluster Containing a Seven Metal Atom Plane. Syntheses and Crystal Structures of [Mn][Mn7(THF)6(CO)12]2, Mn3(THF)2(CO)10, and [Mn(THF)6][Mn(CO)5]2(THF = tetrahydrofuran)." Kong, G.; Harakas, G.N.; Whittlesey, B.R. J. Am. Chem. Soc. 1995, 117, 3502-3509.
- "Preparation of [K] [In{Fe2(CO)8}2] (THF) and Fe2{m-InCl(THF)}2(CO)8 (THF tetrahydrofuran)" Lin, C.-C.; Kong, G.; Cho, C.; Whittlesey, B.R. Inorg. Chem.1993, 32, 2705.
- "Preparation and Crystal Structure of Ru3(m -Cl)2(THF)2(CO)8 (THF = Tetrahydrofuran)" Cho, H.; Whittlesey, B.R. Inorg. Chem.1993, 32, 3789.
Department of Chemistry & Biochemistry
-
Address
1204 Boston Avenue, Lubbock, TX 79409-1061 -
Phone
806.742.3067
