Through combining lipids, enzymes and other substances found in tumors, Indrajit Srivastava believes he has developed an effective way to test fluorescence-guided surgery.
It’s referred to as a phantom, yet it’s anything but scary. In fact, for patients who need tumors extracted, it could help advance a promising surgical procedure.
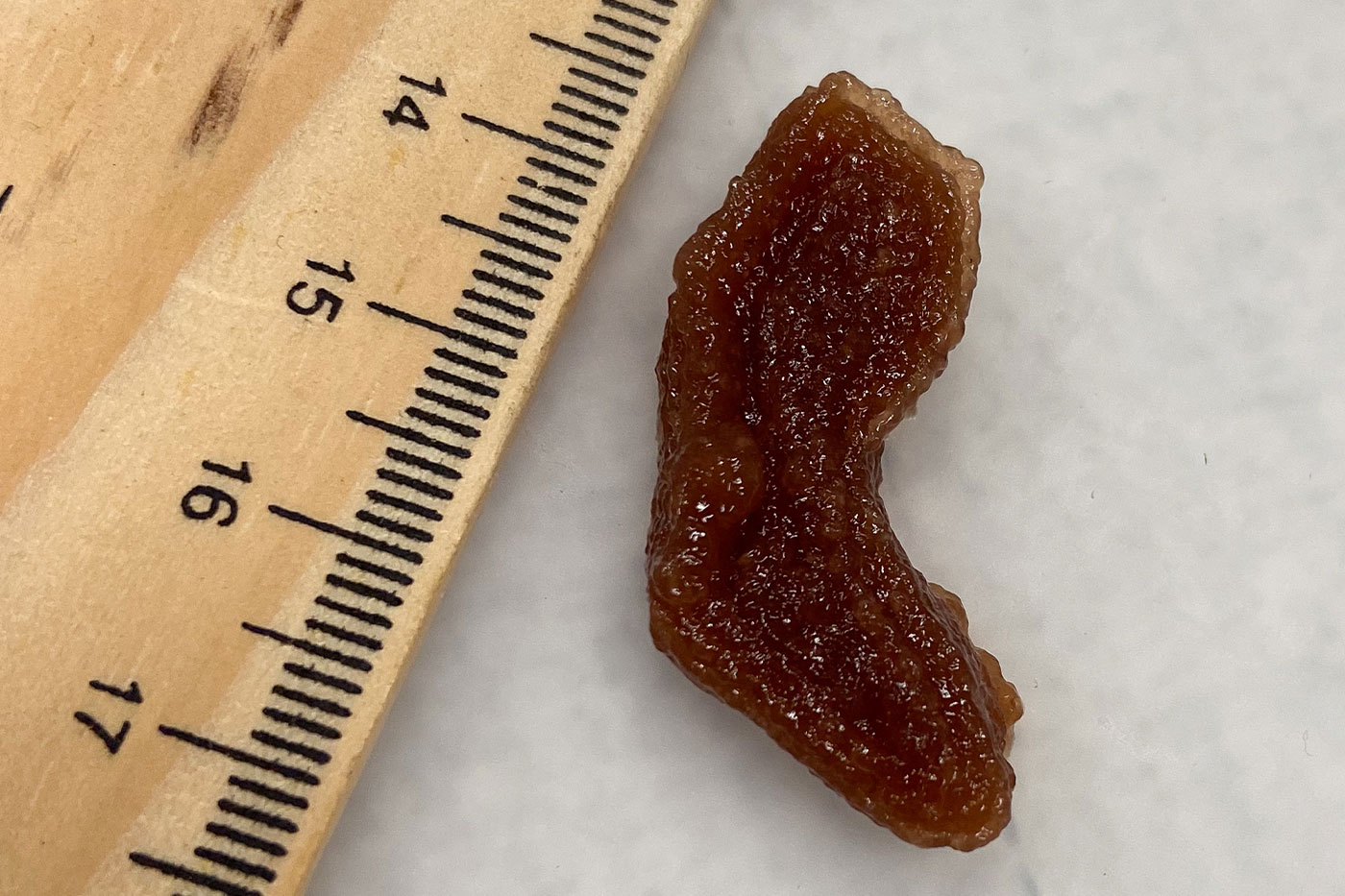
Developed by Department of Mechanical Engineering Assistant Professor Indrajit Srivastava in Texas Tech University’s Edward E. Whitacre Jr. College of Engineering, these 3D phantom models are meant to mimic optical and physical properties of tumors. Roughly a few centimeters long and constructed with substances such as lipids, hemoglobin, enzymes and tumor cells that are bound with gelatin, they are 3D-bioprinted in various shapes of actual tumors.
Srivastava was motivated to create these realistic formations so he could have a better way to test his research in afterglow imaging. He believes this could be a more effective tumor removal process than fluorescence-guided surgery because the light penetrates deeper into tumors and persists longer – up to 10 minutes – allowing surgeons more time for an accurate extraction.


But Srivastava needs to complete more research before afterglow imaging can move closer to widespread adoption, which would likely require testing on mice. Unfortunately, he said this method is unreliable, among other disadvantages.
“As you can expect, mice skin is very thin – only a few millimeters – whereas for humans, it’s very different because we have layers of fats, muscles and tissues that can actually affect the performance of the imaging agent,” Srivastava explained. “So, mice are actually not the best model for these studies.”
This discrepancy prevents many new imaging agents from being used in operating rooms because the tests often do not work well on larger subjects or humans. Srivastava believes his 3D phantom models could eliminate this need to go back to the drawing board, since they incorporate all the elements present in human tumors.


“If a nanoparticle or an imaging agent goes through the artificial tumor, it can actually mimic that environment and its optical signals will be affected like how it would be in human patients,” he said. “This is actually a much cheaper alternative than animal studies because you can do it a lot more to get statistical significance.”
Once Srivastava joined Texas Tech in 2023, recruited by the university for his expertise in surgical technologies, he began work on this project shortly after through The Srivastava Lab. The effort was led by mechanical engineering doctoral student Asma Harun and former biochemistry undergraduate student Nate Bendele.
Other lab members involved in the study were biochemistry undergraduate researchers Isabella Vasquez and Jonathan Djuanda, and mechanical engineering doctoral student Hasnat Rashid. The project also included mini collaborations with other Texas Tech and Texas Tech University Health Sciences Center labs of Gordon Christopher, Joshua Tropp, Paul Egan and Dr. Ulrich Bickel.
After a year-and-a-half of collecting data, the team wrote a paper titled “3D Tumor-Mimicking Phantom Models for Assessing NIR I/II Nanoparticles in Fluorescence-Guided Surgical Interventions” and submitted it for publication in the top-tier journal of the American Chemical Society – ACS Nano.

They were assigned six reviewers – a first for Srivastava – and had a month to complete the recommended revision experiments. That work paid off when nearly all the reviewers loved the artificial tumors concept.
“This is something people have shown interest in,” Srivastava said. “There are a lot of people within Texas Tech who would like to collaborate with us, and now I also have collaborators from Rice University and the University of Texas at San Antonio who want their imaging agents explored and investigated through our phantom models. So that’s exciting for me.”
Srivastava expects to receive similar requests once he presents this work at the Biomedical Engineering Society national meeting in October. To support these collaborations, Srivastava will try to find a binding agent better than gelatin that will more efficiently handle temperature fluctuations faced during the shipping process.
Moving forward, he also will focus on developing a way to keep tumor cells alive longer in the phantom models.


“Once these cells are in the phantom, they slowly start to die,” Srivastava said. “What we’re trying to do is have a phantom where the tumor cells inside stay alive over a period of weeks. Then, when you do imaging, you’re basically trying to image a live tumor and that gives you more optical information that could be useful to a surgeon.”
Srivastava recognizes that the Food and Drug Administration and the National Institutes of Health are moving toward more human-centric studies, and his 3D phantom models could support this transition.
“I think people have overused mice studies at least from the perspective of showcasing their nanoprobes for surgery,” he said. “But at the end of the day, the main priority should be how close the work is to actual clinical use. I think in cases like surgical navigation, my studies are more human centric because we’re trying to address the optical challenges faced by these imaging agents. We’re actually accounting for how this technology would behave when you’re using it in an operating room.”
